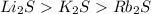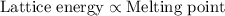Chemistry, 29.06.2019 16:30 dukkchild666
The melting points for the compounds li2s, rb2s, and k2s are 900°c, 530°c, and 840°c, respectively. list these three compounds in order of increasing lattice energy.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
The melting points for the compounds li2s, rb2s, and k2s are 900°c, 530°c, and 840°c, respectively....
Questions in other subjects:


Mathematics, 04.02.2020 20:49

Spanish, 04.02.2020 20:49

Mathematics, 04.02.2020 20:49


Physics, 04.02.2020 20:49




Mathematics, 04.02.2020 20:49