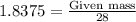The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3si(> 4cr(l) + 3sio2 (s) the reaction is begun with 92.00 g of si and 112.00 g of cr2o3. how many grams of the excess reactant are left after the reaction is complete?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, hannah2718
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2
You know the right answer?
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3...
Questions in other subjects:


Advanced Placement (AP), 23.09.2021 18:10







History, 23.09.2021 18:20

Chemistry, 23.09.2021 18:20

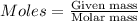 ....(1)
....(1)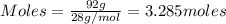
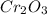
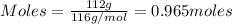
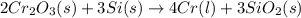
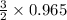 = 1.4475 moles of Silicon.
= 1.4475 moles of Silicon.