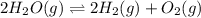Chemistry, 29.06.2019 18:30 johnsonkia873
Consider the following reversible reaction. 2h2o(g)< —> 2h2(g)+o2(g) what is the equilibrium constant expression for the given system?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 11:00, lildestinyquintana
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2

Chemistry, 23.06.2019 14:00, kcutler8603
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 14.0 mol cesium fluoride with 14.0 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
Consider the following reversible reaction. 2h2o(g)< —> 2h2(g)+o2(g) what is the equilibrium c...
Questions in other subjects:


Mathematics, 01.02.2020 00:01


Chemistry, 01.02.2020 00:01






Biology, 01.02.2020 00:01

![k_{eq}=\frac{[H_2]^2[O_2]}{[H_2O]^2}](/tpl/images/1169/7128/9c403.png)