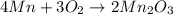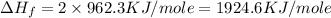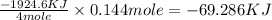Chemistry, 29.06.2019 20:30 ellycleland16
The standard enthalpy of formation of mn2o3 is â962.3 kj/mol. how much heat energy is liberated when 7.9 grams of manganese are oxidized by oxygen gas to mn2o3 at standard state conditions? answer in units of kj.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1

Chemistry, 23.06.2019 12:00, angieplasencia8
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
The standard enthalpy of formation of mn2o3 is â962.3 kj/mol. how much heat energy is liberated when...
Questions in other subjects:

English, 11.10.2020 01:01

Biology, 11.10.2020 01:01





Biology, 11.10.2020 01:01


English, 11.10.2020 01:01

Mathematics, 11.10.2020 01:01

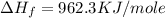 (for 1 mole)
(for 1 mole)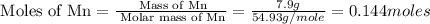
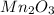 is represented as,
is represented as,