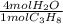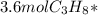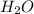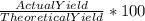Chemistry, 29.06.2019 20:30 jackrider1598
Phease asap given the following equation c3h8 + 5o2 = 3co2 +4h2o if i perform this reaction with 3.6 moles of c3h8 and an excess of oxygen gas , what is my theoretical yeild of water in moles? if i actually isolate 12 moles of waterwhat is my percent yeild?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1


You know the right answer?
Phease asap given the following equation c3h8 + 5o2 = 3co2 +4h2o if i perform this reaction with 3...
Questions in other subjects:

History, 06.01.2021 01:10



Law, 06.01.2021 01:10

Spanish, 06.01.2021 01:10

English, 06.01.2021 01:10


Social Studies, 06.01.2021 01:10

Business, 06.01.2021 01:10

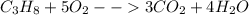
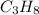 =3.6 mol
=3.6 mol