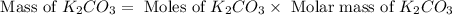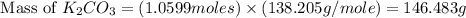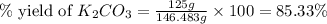Chemistry, 29.06.2019 21:30 vannaht2003
Given the following reaction: 2k3po4 + al2(co3)3 = 3k2co3 + 2alpo4 if i perform this reaction with 150 g of potassium phosphate and 90 g of aluminum carbonate, what is my theoretical yeild in grams of potassium carbonate? if the reaction results in 125 g of potassium carbonate, what is my percent yeild?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Given the following reaction: 2k3po4 + al2(co3)3 = 3k2co3 + 2alpo4 if i perform this reaction with...
Questions in other subjects:










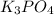 = 150 g
= 150 g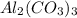 = 90 g
= 90 g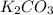 = 138.205 g/mole
= 138.205 g/mole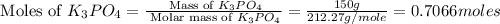
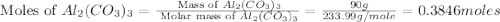
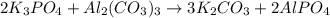
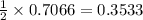 moles of
moles of 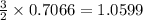 moles of
moles of