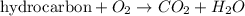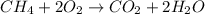Chemistry, 29.06.2019 22:30 strodersage
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2 + 2h2o equation a represents , and equation b represents . blank 1: combustion decomposition double displacement single displacement synthesis blank 2: combustion decomposition double displacement single displacement synthesis

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2...
Questions in other subjects:

Mathematics, 13.01.2021 19:10

History, 13.01.2021 19:10

Mathematics, 13.01.2021 19:10


Physics, 13.01.2021 19:10



Mathematics, 13.01.2021 19:10