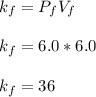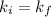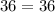Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0 atm, the volume of the gas decreases to 6.0l. find the two constants ki, the initial value of k, and kf, the final value of k, to verify if whether the gas obeys boyle’s law.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0...
Questions in other subjects:


Mathematics, 11.10.2020 09:01

English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01




Physics, 11.10.2020 14:01

Computers and Technology, 11.10.2020 14:01

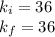
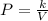
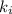 :
: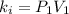
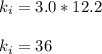
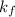 :
: