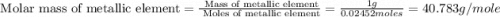1.00 g of a metallic element reacts completely with 300 cm3 of oxygen at 298 k and 1 atm pressure to form an oxide which contains o2– ions. the volume of one mole of gas at this temperature and pressure is 24.0 dm3. what could be the identity of the metal? a calcium b magnesium c potassium d sodium 11

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, MrTeriffic
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1


Chemistry, 23.06.2019 14:30, maelonramirez
William has eight more nickels than dimes in his pocket for a total of $2.50. which equation could be used to determine the number of x dimes in his pocket?
Answers: 1
You know the right answer?
1.00 g of a metallic element reacts completely with 300 cm3 of oxygen at 298 k and 1 atm pressure to...
Questions in other subjects:

English, 03.11.2019 21:31





Mathematics, 03.11.2019 21:31



Biology, 03.11.2019 21:31

Mathematics, 03.11.2019 21:31


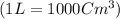


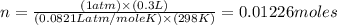
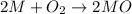
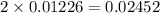 moles of metallic element
moles of metallic element