
Chemistry, 30.06.2019 03:30 whitethunder05
Which of the following solutions will have the highest boiling point? a. 1 molar fecl3 solution b. 1 molar cacl2 solution c. 1 molar kcl solution d. 1 molar nacl solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2


Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

You know the right answer?
Which of the following solutions will have the highest boiling point? a. 1 molar fecl3 solution b....
Questions in other subjects:



History, 31.03.2020 15:17

English, 31.03.2020 15:17




English, 31.03.2020 15:17


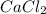 , KCl, and NaCl are all ionic compounds because the bond formation in these compounds is due to the transfer of electrons.
, KCl, and NaCl are all ionic compounds because the bond formation in these compounds is due to the transfer of electrons. 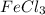 is a covalent compound, that is, it is formed by sharing of electrons. There is weak force of attraction between iron and chlorine atoms thus,
is a covalent compound, that is, it is formed by sharing of electrons. There is weak force of attraction between iron and chlorine atoms thus, 


