Chemistry, 30.06.2019 04:30 rosehayden21
Using the following equation 2c2h6 +7o2 --> 4co2 +6h2o if 9.5g c2h6 react with 130g of o2, how many grams of water will be produced? question options: 32 41 9 17

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Using the following equation 2c2h6 +7o2 --> 4co2 +6h2o if 9.5g c2h6 react with 130g of o2, how ma...
Questions in other subjects:




Mathematics, 02.10.2019 13:00

History, 02.10.2019 13:00

Mathematics, 02.10.2019 13:00

History, 02.10.2019 13:00


Arts, 02.10.2019 13:00

Geography, 02.10.2019 13:00

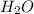 produced will be, 17 grams.
produced will be, 17 grams.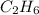 = 9.5 g
= 9.5 g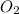 = 130 g
= 130 g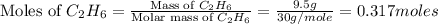
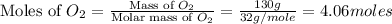
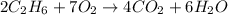
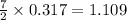 moles of
moles of 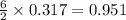 moles of
moles of