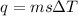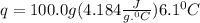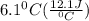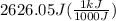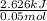A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter. cuso4 (1m) + 2koh (2m) cu(oh)2(s) + k2so4 (0.5m). the temperature of both solutions was 20.2 â°c before mixing and 26.3 â°c after mixing. the heat capacity of the calorimeter is 12.1 j/â°c. assume the specific heat and density of the solution after mixing are the same as those of pure water. from this data, calculate theî´h for the process if there is 0.05 mols of cuso4. (energy of the water + energy of the calorimeter)/(1000 x mol)= kj/mol of reaction

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2


Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter....
Questions in other subjects:

Mathematics, 01.07.2021 08:30

Mathematics, 01.07.2021 08:30


English, 01.07.2021 08:30

Computers and Technology, 01.07.2021 08:30

English, 01.07.2021 08:30

History, 01.07.2021 08:30

Mathematics, 01.07.2021 08:30


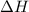 = -
= -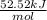
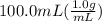
 = 26.3 - 20.2 = 6.1 degree C
= 26.3 - 20.2 = 6.1 degree C