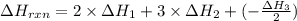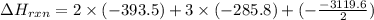Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1


Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1
You know the right answer?
C(graphite) + o2(g) → co2(g)δh o rxn = −393.5 kj/mol h2(g) + 1 2 o2(g) → h2o(l)δh o rxn = −285.8 kj/...
Questions in other subjects:



Mathematics, 18.02.2021 03:50

History, 18.02.2021 03:50

Physics, 18.02.2021 03:50




History, 18.02.2021 03:50


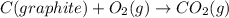
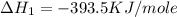
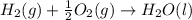
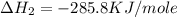
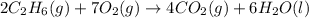
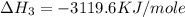
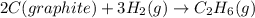
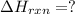
![2\times eq.(1)+\frac{1}{2}(eq.3)\text{[reversing equation 3 and dividing it by 2]}+3(eq.2)](/tpl/images/0034/7108/524c9.png)