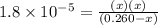Chemistry, 30.06.2019 12:00 twinkieslayer
Hc2h3o2(aq)+h2o(l)⇌h3o+(aq)+c2h3o−2 (aq) kc=1.8×10−5 at 25∘c part a if a solution initially contains 0.260 m hc2h3o2, what is the equilibrium concentration of h3o+ at 25∘c? express your answer using two significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Hc2h3o2(aq)+h2o(l)⇌h3o+(aq)+c2h3o−2 (aq) kc=1.8×10−5 at 25∘c part a if a solution initially contains...
Questions in other subjects:

Mathematics, 27.06.2020 02:01




Mathematics, 27.06.2020 02:01

Chemistry, 27.06.2020 02:01

Mathematics, 27.06.2020 02:01


Mathematics, 27.06.2020 02:01

 at
at 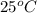 is,
is,  .
.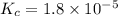
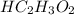 = 0.260 m
= 0.260 m
![K_c=\frac{[H_3O^+][C_2H_3O_2^-]}{[HC_2H_3O_2]}](/tpl/images/0034/7668/e70e1.png)