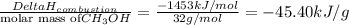Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, gizmo50245
Calculate the mass percent of hydrogen in methyl acetate
Answers: 1

Chemistry, 21.06.2019 16:30, KieraKimball
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Methanol (ch3oh) has been proposed as an alternative fuel. calculate the standard enthalpy of combus...
Questions in other subjects:


History, 12.03.2021 22:10


Mathematics, 12.03.2021 22:10

History, 12.03.2021 22:10


Mathematics, 12.03.2021 22:20



Mathematics, 12.03.2021 22:20








![=[2\times (-393.5 kJ/mol)+4\times (-286 kJ/mol)]-[2\times (-239 kJ/mol)+3\times (0 kJ/mol)]=-1453 kj/mol](/tpl/images/0035/4633/40853.png)