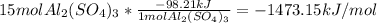Chemistry, 30.06.2019 19:00 lilchannelll4125
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance the reaction. calculate the total enthalpy change that would occur from 15 moles of al2(so4)3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance...
Questions in other subjects:



Mathematics, 11.11.2020 06:40



History, 11.11.2020 06:40






![H_{reaction}^{0}=[H_{f}^{0}(Al_{2}O_{3}(s)) + (3*H_{f}^{0}(H_{2}SO_{4}(aq))] - [H_{f}^{0}(Al_{2}SO_{4}(aq)) + (3*H_{f}^{0}(H_{2}O(l))]](/tpl/images/0035/8926/80689.png)
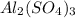 reacts will be=
reacts will be=