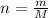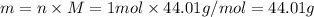Chemistry, 30.06.2019 19:00 beastboy13
The molar mass of co2 is 44.01 g/mole. choose which of the following correctly represents this relationship correctly as a conversion factor. 1 mol co2 = 44.01 g co2 44.01 mol co2 = 1 g co2 44.01 molecules co2 = 1 g co2 1 molecule co2 = 44.01 g co2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
The molar mass of co2 is 44.01 g/mole. choose which of the following correctly represents this relat...
Questions in other subjects:

Mathematics, 14.07.2019 07:00

English, 14.07.2019 07:00

English, 14.07.2019 07:00

Mathematics, 14.07.2019 07:00


Health, 14.07.2019 07:00




Mathematics, 14.07.2019 07:00

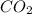 = 44.01 grams of
= 44.01 grams of