
Chemistry, 01.07.2019 03:00 stephanieyingepbtcf8
Dilute aqueous hydrogen peroxide is used as a bleaching agent and for disinfecting surfaces and small cuts. its concentration is sometimes given as a certain number of "volumes hydrogen peroxide," which refers to the number of volumes of o2 gas, measured at stp, that a given volume of hydrogen peroxide solution will release when it decomposes to o2 and liquid h2o. how many grams of hydrogen peroxide are in 0.125−l of 20 "volumes hydrogen peroxide" solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, mildredelizam
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 21.06.2019 20:30, Garciaapril1597
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2
You know the right answer?
Dilute aqueous hydrogen peroxide is used as a bleaching agent and for disinfecting surfaces and smal...
Questions in other subjects:










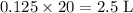 of oxygen gas.
of oxygen gas. here is measured under STP, which means that every
here is measured under STP, which means that every  of gas would have a volume of
of gas would have a volume of  . There would thus be
. There would thus be  of molecules in the 2.5 L of
of molecules in the 2.5 L of 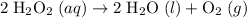
 , meaning that each mole of it would have a mass of 34.02 grams. 0.224 mol of
, meaning that each mole of it would have a mass of 34.02 grams. 0.224 mol of  would thus have a mass of
would thus have a mass of 



