
Chemistry, 01.07.2019 08:30 edeliz2804
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced when 65.2 g of al is reacted with an excess (unlimited) supply of fe2o3?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2


Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced...
Questions in other subjects:



History, 05.08.2019 01:30







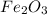 .
.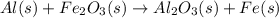


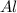 reacts with one mole of
reacts with one mole of 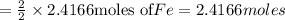
 produced =
produced =


