Chemistry, 01.07.2019 09:00 jinjuwdjesus
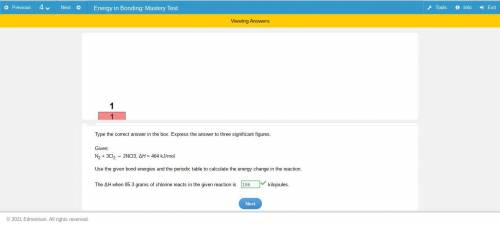
Given: n2 + 3cl2 → 2ncl3, δh = 464 kj/mol use the given bond energies and the periodic table to calculate the energy change in the reaction. the δh when 85.3 grams of chlorine reacts in the given reaction is kj answer must be in 3 sig fig!

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3


Chemistry, 23.06.2019 09:30, Adolfosbaby
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
Given: n2 + 3cl2 → 2ncl3, δh = 464 kj/mol use the given bond energies and the periodic table to cal...
Questions in other subjects:




Mathematics, 29.08.2019 04:10

Mathematics, 29.08.2019 04:10

History, 29.08.2019 04:10




History, 29.08.2019 04:10