
Chemistry, 01.07.2019 09:00 brianadee800
How is the enthalpy of reaction shown in this potential energy diagram? a. as the sum of the energy of the products and the energy of the reactants b. as the sum of the activation energy and the energy of the products c. as the difference of the energy of the reactants and the energy of the products d. as the difference of the activation energy and the energy of the products
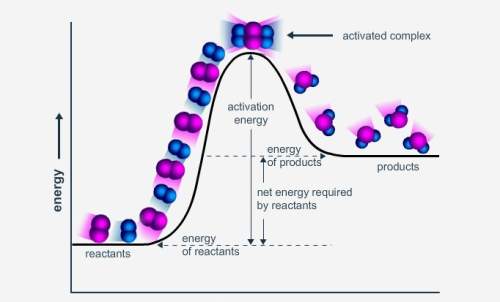

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kylieweeks052704
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2
You know the right answer?
How is the enthalpy of reaction shown in this potential energy diagram? a. as the sum of the energy...
Questions in other subjects:

Social Studies, 12.11.2020 23:40

Mathematics, 12.11.2020 23:40

Mathematics, 12.11.2020 23:40

Mathematics, 12.11.2020 23:40


Health, 12.11.2020 23:40




English, 12.11.2020 23:40

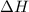 .
.

