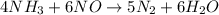For the reaction nh3 + no → n2 + h2o, identify the reactants, products, and their coefficients once the equation is balanced. reactants: 2nh3 and 2no; products: 2n2 and 3h2o products: 2nh3 and 3no; reactants: 2n2 and 3h2o reactants: 4nh3 and 6no; products: 5n2 and 6h2o products: 4nh3 and 2no; reactants: 3n2 and 6h2o

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 20:30, Willywill15
If 4.88 grams of zn react with 5.03 grams of s8 to produce 6.02 grams of zns, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem. unbalanced equation: zn + s8 yields zns
Answers: 3

Chemistry, 24.06.2019 02:20, josueur9656
Magnetic inks are made by mixing very small particles of a magnetic substance with a liquid. what are the uses of magnetic inks.
Answers: 1
You know the right answer?
For the reaction nh3 + no → n2 + h2o, identify the reactants, products, and their coefficients once...
Questions in other subjects:

Mathematics, 15.02.2021 21:30

History, 15.02.2021 21:30

Mathematics, 15.02.2021 21:30

Mathematics, 15.02.2021 21:30

Mathematics, 15.02.2021 21:30

Biology, 15.02.2021 21:30


Chemistry, 15.02.2021 21:30

History, 15.02.2021 21:30

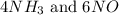
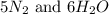
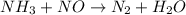
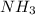 , the coefficient '6' put before the
, the coefficient '6' put before the 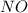 , the coefficient '5' put before the
, the coefficient '5' put before the 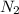 and the coefficient '6' put before the
and the coefficient '6' put before the 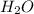 and then we get the balanced chemical equation.
and then we get the balanced chemical equation.