
Chemistry, 01.07.2019 11:00 lisacarter0804
Calculate the mass of 25,000 molecules of nitrogen gas. (1 mole = 6.02 x 1023 molecules)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2


Chemistry, 23.06.2019 15:30, kfull6027
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3 +(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1
You know the right answer?
Calculate the mass of 25,000 molecules of nitrogen gas. (1 mole = 6.02 x 1023 molecules)...
Questions in other subjects:



Biology, 21.05.2020 06:01



Biology, 21.05.2020 06:01

Mathematics, 21.05.2020 06:01

Mathematics, 21.05.2020 06:01

Chemistry, 21.05.2020 06:01

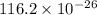 grams
grams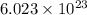 of particles.
of particles.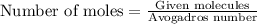
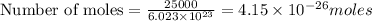
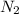 gas weighs = 28 grams
gas weighs = 28 grams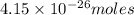 of
of 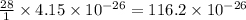 grams
grams


