
Chemistry, 01.07.2019 11:00 ptonygonzalez701
Calculate the ph at the equivalence point for the titration of 0.230 m methylamine (ch3nh2) with 0.230 m hcl. the kb of methylamine is 5.0× 10–4.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Calculate the ph at the equivalence point for the titration of 0.230 m methylamine (ch3nh2) with 0.2...
Questions in other subjects:


Biology, 05.07.2019 23:00

English, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00


Mathematics, 05.07.2019 23:00


Mathematics, 05.07.2019 23:00

Computers and Technology, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00

![[OH^-]](/tpl/images/0038/4745/b2910.png) be x
be x![[CH3NH_2]](/tpl/images/0038/4745/f7563.png) , c = 0.230 M
, c = 0.230 M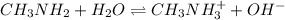
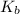 :
:![K_b=\frac{[CH_3NH_3^+][+OH^-]}{[CH_3NH_2]}=\frac{x\times x}{c-x}=\frac{x^2}{c-x}](/tpl/images/0038/4745/3e9a4.png)
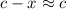 .
.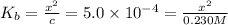
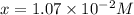
![[H^+]](/tpl/images/0038/4745/07acb.png) = 0.230 M
= 0.230 M![[OH^-]=[H^+]](/tpl/images/0038/4745/2bc09.png) will result in neutral solution, since
will result in neutral solution, since ![[OH^-]](/tpl/images/0038/4745/9eb9b.png)
![[H^+]_{\text{left in solution}}=[H^+]-[OH^-]=0.230-1.07\times 10^{-2}=0.2193 M](/tpl/images/0038/4745/6ec30.png)
![pH=-log{[H^+]_{\text{left in solution}}=-log(0.2193)=0.65](/tpl/images/0038/4745/0225f.png)


