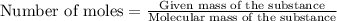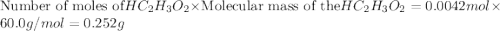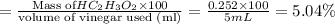The formula to determine the percent of hc2h3o2 (mass/volume) in vinegar is percent (m/v) = (grams of hc2h3o2/ volume of vinegar used (ml) ) x 100. if 5.0 ml of vinegar were used for the titration and 0.0042 moles of hc2h3o2 were required to reach the endpoint, calculate the percent of hc2h3o2 in vinegar. the molar mass of hc2h3o2 is 60.0 g / mol. question 10 options:

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
The formula to determine the percent of hc2h3o2 (mass/volume) in vinegar is percent (m/v) = (grams o...
Questions in other subjects:



History, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


Advanced Placement (AP), 18.03.2021 03:10


History, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10

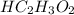 in vinegar 5.04%.
in vinegar 5.04%.