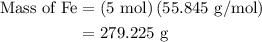Chemistry, 01.07.2019 13:00 amycressey1970
The following data was collected when a reaction was performed experimentally in the laboratory. fe2o3 al al2o3 fe starting amount in reaction 3 moles 5 moles ? ? determine the maximum amount of fe that was produced during the experiment. explain how you determined this amount.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kylieweeks052704
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 21.06.2019 13:50, 24lbriscoe
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1


Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
The following data was collected when a reaction was performed experimentally in the laboratory. fe2...
Questions in other subjects:




English, 04.05.2021 07:20

Mathematics, 04.05.2021 07:20

English, 04.05.2021 07:20

Mathematics, 04.05.2021 07:20


French, 04.05.2021 07:20

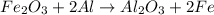
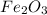 = 3 moles
= 3 moles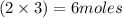 of Al
of Al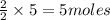 of Fe.
of Fe.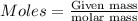
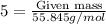
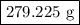 of Fe is produced during the given experiment.
of Fe is produced during the given experiment.
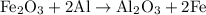
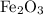 reacts with two moles of Al to produce one mole of
reacts with two moles of Al to produce one mole of 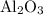 and two moles of Fe.
and two moles of Fe.
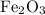 and 5 moles of Al. Therefore the amount of Fe produced by 3 moles of
and 5 moles of Al. Therefore the amount of Fe produced by 3 moles of 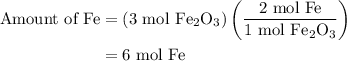
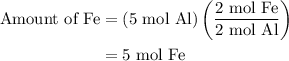
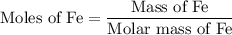 ...... (1)
...... (1) 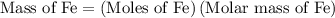 ...... (2)
...... (2)