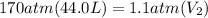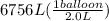Chemistry, 01.07.2019 17:30 2020davidhines
Acylinder containing 44.0 l of helium gas at a pressure of 170 atm is to be used to fill toy balloons to a pressure of 1.1 atm. each inflated balloon has a volume of 2.0 l. what is the maximum number of balloons that can be inflated? (remember that 44.0 l of helium at 1.1 atm pressure will remain in the 'exhausted' cylinder) round your answer to two significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Acylinder containing 44.0 l of helium gas at a pressure of 170 atm is to be used to fill toy balloon...
Questions in other subjects:



Mathematics, 27.04.2021 20:50


English, 27.04.2021 20:50


Chemistry, 27.04.2021 20:50


Mathematics, 27.04.2021 20:50


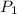 = 170 atm
= 170 atm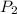 = 1.1 atm
= 1.1 atm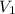 = 44.0 L
= 44.0 L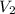 = ?
= ?