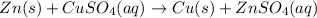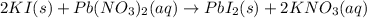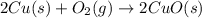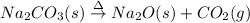Chemistry, 02.07.2019 01:00 farashka03
Lab: types of reactions student guide data record your data either in your lab notebook or in the space below. reaction 1~ add zinc to copper sulfate observations of reactants: predicted type(s) of reaction: observations of products: balanced chemical equation: → (s) + ) type(s) of reaction: reaction 2~ mix potassium iodide and lead (ii) nitrate observations of reactants: predicted type(s) of reaction: observations of products: balanced chemical equation: → (s) + ) type(s) of reaction: reaction 3~ burn copper wire observations of reactants: predicted type(s) of reaction: observations of products: balanced chemical equation: ) + ) ⟶ (s) type(s) of reaction: reaction 4~ heat sodium carbonate observations of reactants: predicted type(s) of reaction: observations of products: balanced chemical equation: ) ⟶ (s) + co2(g) type(s) of reaction:

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
You know the right answer?
Lab: types of reactions student guide data record your data either in your lab notebook or in the s...
Questions in other subjects:


History, 15.07.2019 21:30


Mathematics, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30