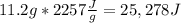Chemistry, 02.07.2019 02:00 culturedxnat
Water’s heat of vaporization is 2,257 joules/gram. how much energy is released when 11.2 grams of water vapor condenses into liquid? a. 202 j b. 2,246 j c. 3,840 j d. 9,950 j e. 25,300 j

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 10:30, robot2998
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1
You know the right answer?
Water’s heat of vaporization is 2,257 joules/gram. how much energy is released when 11.2 grams of wa...
Questions in other subjects:


SAT, 18.02.2022 14:00

Mathematics, 18.02.2022 14:00




Mathematics, 18.02.2022 14:00

Mathematics, 18.02.2022 14:00

History, 18.02.2022 14:00

 :
: