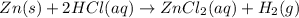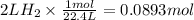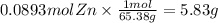How many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to the chemical equation shown below? how many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to the chemical equation shown below? 5.83 g 11.7 g 0.171 g 131 g?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
How many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to...
Questions in other subjects:

Biology, 11.05.2021 16:00

Arts, 11.05.2021 16:00



Spanish, 11.05.2021 16:00