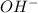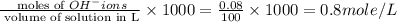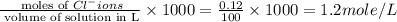Chemistry, 02.07.2019 03:00 stefanylopez731
Calculate the molarity of each type of ion remaining in solution after 20.0 ml of 6.00 m hcl is mixed with 50.0 ml of 2.00 m barium hydroxide and 30.0 ml of water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Calculate the molarity of each type of ion remaining in solution after 20.0 ml of 6.00 m hcl is mixe...
Questions in other subjects:

Mathematics, 15.10.2019 21:00





Social Studies, 15.10.2019 21:00


Health, 15.10.2019 21:00


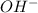 ions = 0.8 mole/L
ions = 0.8 mole/L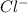 ions = 1.2 mole/L
ions = 1.2 mole/L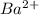 ions = 1 mole/L
ions = 1 mole/L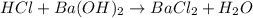
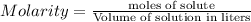
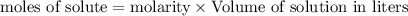
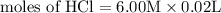 = 0.12
= 0.12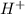 and 1 mole of
and 1 mole of 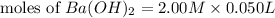 = 0.1
= 0.1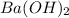 dissociates to give 2 moles of
dissociates to give 2 moles of