
Which of the following statements is true about the strength of the intermolecular forces in ch4 and nh3? a: ch4 ≥ nh3 because ch4 is tetrahedral but nh3 is pyramidal. b: ch4 < nh3 because δ− on c in the ch bond is greater than δ− on n in the nh bond. c: ch4 < nh3 because the nh bond is more polar than the ch bond. d: ch4 ≥ nh3 because ch4 has h bonding but nh3 has dispersion forces.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 23.06.2019 08:00, IntellTanito
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2

Chemistry, 23.06.2019 09:30, crawford184232323234
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2

Chemistry, 23.06.2019 16:30, tiffanibell71
Two like-charged particles are placed close to each other. how would the force of repulsion be affected if the charge on one of the particles is doubled and that on the other is reduced to half the original value?
Answers: 1
You know the right answer?
Which of the following statements is true about the strength of the intermolecular forces in ch4 and...
Questions in other subjects:

Mathematics, 06.07.2019 13:00


Mathematics, 06.07.2019 13:00


English, 06.07.2019 13:00


History, 06.07.2019 13:00

History, 06.07.2019 13:00


Mathematics, 06.07.2019 13:00

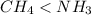 because the NH bond is more polar than CH bond.
because the NH bond is more polar than CH bond.

