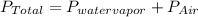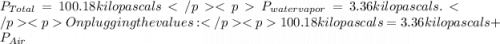Chemistry, 02.07.2019 05:30 heavenwagner
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the total pressure is 100.18 kilopascals, and the partial pressure of the water vapor is 3.36 kilopascals. what is the partial pressure of the air in the sample? a. 29.8 kpa b. 51.77 kpa c. 96.82 kpa d. 103.54 kpa e. 337 kpa

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 13:00, Crxymia
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
You know the right answer?
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the...
Questions in other subjects:

English, 26.05.2021 18:00

English, 26.05.2021 18:00


English, 26.05.2021 18:00




English, 26.05.2021 18:00