

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Water forms according to the equation below: 2h2(g) + o2(g) jpg 2h2o(g) hrxn = -483.64 kj how much...
Questions in other subjects:


Mathematics, 18.01.2020 17:31

Mathematics, 18.01.2020 17:31



Mathematics, 18.01.2020 17:31

Mathematics, 18.01.2020 17:31


Mathematics, 18.01.2020 17:31

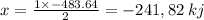


 released amount of energy = -483.64 KJ
released amount of energy = -483.64 KJ


