
Chemistry, 02.07.2019 12:30 geminigirl077
Ahydrogen-like ion is an ion containing only one electron. the energy of the electron in a hydrogen-like ion is given by en = ? (2.18 × 10? 18j) z2 ( 1 n2 ) where n is the principal quantum number and z is the atomic number of the element. plasma is a state of matter consisting of positive gaseous ions and electrons. in the plasma state, a mercury atom could be stripped of its 80 electrons and therefore could exist as hg80+. use the equation above to calculate the energy required for the last ionization step: hg79+(g) ? hg80+(g)+ e?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Ahydrogen-like ion is an ion containing only one electron. the energy of the electron in a hydrogen-...
Questions in other subjects:




English, 04.08.2020 20:01







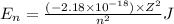
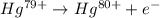
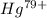 is
is 
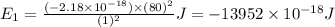
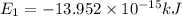 (Conversion Factor: 1kJ = 1000J)
(Conversion Factor: 1kJ = 1000J)

