
Chemistry, 02.07.2019 15:30 Jordan0423
How many moles of potassium chloride and oxygen can be produced from 100.0g of potassium chlorate? the balanced equation is: 2 kclo3=2kcl+3o2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


You know the right answer?
How many moles of potassium chloride and oxygen can be produced from 100.0g of potassium chlorate?...
Questions in other subjects:

Social Studies, 31.08.2019 08:30

Mathematics, 31.08.2019 08:30


Mathematics, 31.08.2019 08:30



Health, 31.08.2019 08:30


Mathematics, 31.08.2019 08:30

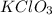 = 100.0 g
= 100.0 g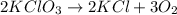
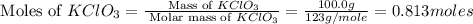
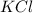 and
and 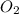 .
.

