
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the molecules. carbon tetrachloride ccl4 is also nonploar but is made larger molecules that are more strongly affected by london dispersion forces. based on this information cs2 likely has a melting point and a vapor pressure when compared with ccl4

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the mole...
Questions in other subjects:

Chemistry, 21.04.2021 23:50


Mathematics, 21.04.2021 23:50


English, 21.04.2021 23:50


Mathematics, 21.04.2021 23:50



Mathematics, 21.04.2021 23:50

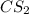 where as the vapor pressure of
where as the vapor pressure of ![CS_2[/tex[ is higher than [tex]CCl_4](/tpl/images/0043/9801/99b5e.png) .
.

