
Chemistry, 02.07.2019 22:00 ethanm2685
Question 30select the correct answer from each drop-down menu. a reaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is the equation of the reaction: mg(clo3)2 + 2naoh → mg(oh)2 + 2naclo3. determine the theoretical amount of each product that the reaction will produce. the reaction will produce of magnesium hydroxide and of sodium chlorate. (both have the same dropdown) a. 1.36 b. 1.57 c. 2.72 d. 3.14 e. 4.52 (all in moles)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

You know the right answer?
Question 30select the correct answer from each drop-down menu. a reaction proceeds with 2.72 moles o...
Questions in other subjects:

French, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00




English, 16.04.2021 14:00


Mathematics, 16.04.2021 14:00

English, 16.04.2021 14:00


 react with 2 mole of
react with 2 mole of 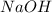
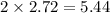 moles of
moles of  and
and  by using the moles of limiting reagent.
by using the moles of limiting reagent. moles of
moles of 

