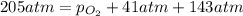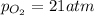Chemistry, 03.07.2019 01:00 FailingstudentXD
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres. the partial pressure of nitrogen is 143 atmospheres, and the partial pressure of helium is 41 atmospheres. what is the partial pressure of oxygen in the tank? a. 21 atm b. 103 atm c. 307 atm d. 389 atm

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3


Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres...
Questions in other subjects:

History, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01

Social Studies, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01



History, 24.08.2020 19:01

Arts, 24.08.2020 19:01

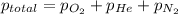
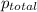 = 205 atm
= 205 atm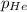 = 41 atm
= 41 atm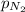 = 143 atm
= 143 atm