
Chemistry, 03.07.2019 01:30 idontknow11223344
Using iupac nomenclature, give the name of bh3 and alh3 and elaborate why that name is correct. a) boron trihydride & aluminum trihydride - both molecules combine two non-metals and form covalent bonds. b) boron hydride & aluminum hydride - both compounds combine a main group metal and a non-metal to form ionic bonds. c) boron trihydride - boron and hydrogen are both non-metals and form covalent bonds. aluminum hydride - aluminum is a main group metal and hydrogen is a non-metal and they form ionic bonds. d) boron hydride - boron is a main group metal and hydrogen is a non-metal and they form ionic bonds. aluminum trihydride - aluminum and hydrogen are both non-metals and form covalent bonds.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Using iupac nomenclature, give the name of bh3 and alh3 and elaborate why that name is correct. a)...
Questions in other subjects:



Mathematics, 15.04.2020 19:11





Mathematics, 15.04.2020 19:11


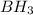 is a covalent compound because sharing of electrons takes place between hydrogen and boron. Both the elements are non-metals and hence, will form covalent bond.
is a covalent compound because sharing of electrons takes place between hydrogen and boron. Both the elements are non-metals and hence, will form covalent bond.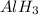 is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.


