Chemistry, 03.07.2019 02:00 710jonathan
Calculate the ph of the solution made by adding 0.50 mol of hobr and 0.30 mol of kobr to 1.00 l of water. the value of ka for hobr is 2.0ă—10â’9. express your answer numerically using two decimal places.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, TheRealKodakBlack
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

You know the right answer?
Calculate the ph of the solution made by adding 0.50 mol of hobr and 0.30 mol of kobr to 1.00 l of w...
Questions in other subjects:



Mathematics, 12.01.2021 03:10

Biology, 12.01.2021 03:10

Mathematics, 12.01.2021 03:10

Mathematics, 12.01.2021 03:10

Mathematics, 12.01.2021 03:10


History, 12.01.2021 03:10

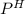 of the solution containing HOBr and KOBr.
of the solution containing HOBr and KOBr.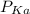 + log
+ log ![\frac{[salt]}{[acid]}](/tpl/images/0044/6662/a0d10.png)
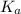 value of HOBr is 2×10⁻⁹.
value of HOBr is 2×10⁻⁹.