
Chemistry, 03.07.2019 04:00 guzmangisselle
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are not easily added to molecules with single bonds. b. atoms are not easily added to molecules with double bonds. c. saturated hydrocarbons have fewer carbon atoms than unsaturated hydrocarbons.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mamasmontoya
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2



Chemistry, 23.06.2019 11:30, angelicar1160
Which of the following is a property of an acid solution? a. slippery to the touch b. ph less than 7 c. turns red litmus paper blue d. bitter taste
Answers: 1
You know the right answer?
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are...
Questions in other subjects:


Mathematics, 12.11.2020 04:00

Mathematics, 12.11.2020 04:00

Health, 12.11.2020 04:00


Chemistry, 12.11.2020 04:00



Mathematics, 12.11.2020 04:00

Spanish, 12.11.2020 04:00

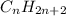
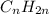 and
and 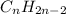
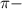 bonds. They are weaker than
bonds. They are weaker than 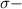 bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having
bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having 

