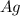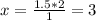Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

You know the right answer?
If 1.5 moles of copper metal react with 4.0 moles of silver nitrate, how many moles of silver metal...
Questions in other subjects:


Mathematics, 20.12.2020 01:40

Mathematics, 20.12.2020 01:40






Mathematics, 20.12.2020 01:40

 will be left.
will be left.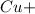 2
2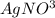 →
→ 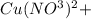 2
2