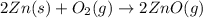Chemistry, 03.07.2019 11:30 vlactawhalm29
When zinc metal reacts with oxygen gas, 2zn(s) + o2(g) → 2zno(g), large amounts of light and heat are released. a student states that this reaction is a combustion reaction but not a redox reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction. asap

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
When zinc metal reacts with oxygen gas, 2zn(s) + o2(g) → 2zno(g), large amounts of light and heat ar...
Questions in other subjects:


English, 16.09.2019 02:00




Mathematics, 16.09.2019 02:00


Spanish, 16.09.2019 02:00