
Chemistry, 03.07.2019 11:30 lobatospitones
In this reaction, how does the rate of forward reaction vary with the concentration of the product? 2h2s(g) ⇌ 2h2(g) + s2(g) it increases with an increase in the concentration of s2(g). it decreases with a decrease in the concentration of h2(g). it increases with a decrease in the concentration of h2(g). it decreases with an increase in the concentration of s2(g). it decreases with increase in the concentration of h2(g). multiple answers

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
In this reaction, how does the rate of forward reaction vary with the concentration of the product?...
Questions in other subjects:

Mathematics, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00


Chemistry, 30.11.2020 22:00

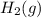 . D) It decreases with increase in the concentration of
. D) It decreases with increase in the concentration of 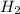 or
or 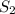 is increased, the reaction will shift in backward direction and the rate of forward direction decreases.
is increased, the reaction will shift in backward direction and the rate of forward direction decreases.

