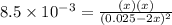Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
You know the right answer?
For the equilibrium 2 ibr (g) i2 (g) + br2 (g) kp=8.5 ×10-3 at 150 oc. if 0.025 atm of ibr is place...
Questions in other subjects:

Mathematics, 26.04.2021 14:00


Mathematics, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00


Mathematics, 26.04.2021 14:00

English, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00

Chemistry, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00

 and
and  is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.
is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.
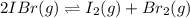
![K_p=\frac{[P_I_2][P_{Br}_2]}{[P_{IBr}]^2}](/tpl/images/0046/9546/983fc.png)