
Two solutions namely, 500 ml of 0.50 m hcl and 500 ml of 0.50 m naoh at the same temperature of 21.6 are mixed in a constant-pressure calorimeter. the heat capacity of the calorimeter was 450 j/c. given that the specific heat of the solution is 4.184 j/gc, the density of the solution is 1.0 g/ml, and that the heat of neutralization for the process h+oh=h2o is -56.2 kj, what is the final temperature of the mixed solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2



Chemistry, 23.06.2019 21:20, wsdafvbhjkl
Consider the total ionic equation below. what are the spectator ions in this equation? h+ and oh- h+ and ba2+cro2-4 and oh- cro2-4 and ba2+
Answers: 3
You know the right answer?
Two solutions namely, 500 ml of 0.50 m hcl and 500 ml of 0.50 m naoh at the same temperature of 21.6...
Questions in other subjects:


Mathematics, 28.11.2020 23:50

Biology, 28.11.2020 23:50

Physics, 28.11.2020 23:50

History, 28.11.2020 23:50

English, 28.11.2020 23:50



English, 28.11.2020 23:50

English, 28.11.2020 23:50

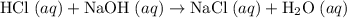
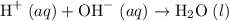
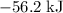 . Thus the combination of every mole of hydrogen ions and hydroxide ions in solution would produce
. Thus the combination of every mole of hydrogen ions and hydroxide ions in solution would produce 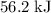 or
or 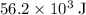 of energy.
of energy.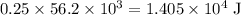 of energy.
of energy. 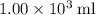 of the 1.0 gram per milliliter solution. Accordingly, it would have a mass of
of the 1.0 gram per milliliter solution. Accordingly, it would have a mass of  .
. 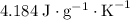 . The solution thus have a heat capacity of
. The solution thus have a heat capacity of 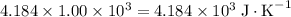 . Note that one degree Kelvins K is equivalent to one degree celsius ℃ in temperature change measurements.
. Note that one degree Kelvins K is equivalent to one degree celsius ℃ in temperature change measurements.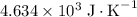 , meaning that its temperature would rise by 1 degree celsius on the absorption of 4.634 × 10³ joules of energy.
, meaning that its temperature would rise by 1 degree celsius on the absorption of 4.634 × 10³ joules of energy.  are available from the reaction. Thus, the temperature of the system shall have risen by 3.03 degrees celsius to 24.6 degrees celsius by the end of the reaction.
are available from the reaction. Thus, the temperature of the system shall have risen by 3.03 degrees celsius to 24.6 degrees celsius by the end of the reaction.

