
Chemistry, 03.07.2019 17:30 enitramedouard12
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane needed to produce 90.9 g of carbon dioxide. calculate the mass of butane needed to produce 90.9 g of carbon dioxide.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane ne...
Questions in other subjects:

Mathematics, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00

History, 27.07.2019 03:00

Mathematics, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00

Health, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00

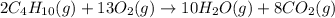
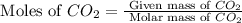
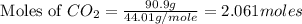
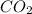 produced from 2 moles
produced from 2 moles 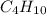
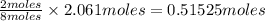 of
of 

