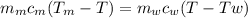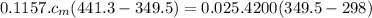Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water initially at 25.0°c and allowed to reach thermal equilibrium. the final temperature of the system is 76.5°c. what is the identity of the unknown substance? assume no heat is lost to the surroundings

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Dreambig85
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2


Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water init...
Questions in other subjects:



English, 16.01.2020 00:31



Mathematics, 16.01.2020 00:31



Spanish, 16.01.2020 00:31

 .
.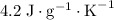 and a density of
and a density of 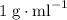 . 25.0 milliliters of water thus has a mass of 25.0 grams.
. 25.0 milliliters of water thus has a mass of 25.0 grams. 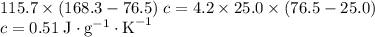
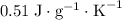 . This substance is thus probably steel.
. This substance is thus probably steel.