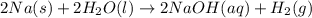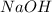Chemistry, 04.07.2019 07:00 Robinlynn228
Assign an oxidation number to each atom in the products. 2na(s)+2h2o(l)→2naoh(aq)+h2(g) assign an oxidization number for: na , o , h (in naoh) , h (in h2) i'm not sure how to assign oxidization numbers, if someone could explain it to me that'd be great! i have an exam that involves oxidization numbers coming up, and i'm completely clueless on them! you in advance!

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
Assign an oxidation number to each atom in the products. 2na(s)+2h2o(l)→2naoh(aq)+h2(g) assign an ox...
Questions in other subjects:

Arts, 03.03.2021 23:30



Chemistry, 03.03.2021 23:30


Mathematics, 03.03.2021 23:30

History, 03.03.2021 23:30


Engineering, 03.03.2021 23:30

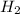 is zero.
is zero.